Inflammatory Bowel Diseases
Cellvizio® is the real-time in vivo cellular imaging platform that allows you to observe, understand, and predict relapse and remission cycles while managing Inflammatory Bowel Disease. (IBD)
Under current protocol, 30% of IBD patients relapse every year.1,2
52% of Ulcerative Colitis (UC) patients who have an active disease are included in this statistic.1 As a result, 187,000 hospitalizations per year, take place, specifically for Crohn's disease (CD) in the US.3 Current disease assessment techniques are suboptimal and do not accurately predict long term prognosis.4
Cellvizio enables you to determine the optimal course of action for IBD with the knowledge of point-in-time reactions as they happen in real-time.
Cellvizio® Clinical Value
With Cellvizio®, physicians predict relapse within the next 12 months, as well as major clinical events that require hospitalization or surgery for IBD patients.5,6
It also allows identification of residual colonic inflammation not detectable macroscopically in patients with IBD which in turn can help in determining the need for additional anti-inflammatory therapy and allows prediction of clinical outcomes.12
As a result, physicians are able to monitor treatment to differentiate responders from non-responders for UC patients.7
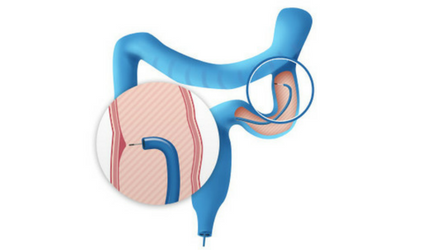
"Cellvizio® is the only technology that allows us to see functional healing, and it is worth having this information as it is the best determinant of outcome for my IBD patients"
-- Prof. Dr. T. Rath, University Hospital Erlangen
Patient Management
With Cellvizio®, patient management is improved because physicians can now differentiate between diagnoses of IBD (92% sensitivity, 91% specificity).8 They can also predict relapse and enable tailored biologic therapy to reduce IBD-related hospitalizations.9 During a flare period, mucosal inflammation is confirmed.10 The treatment improves because physicians can assess mucosal barrier function for predicting improved long-term patient outcomes for UC patients.7 Furthermore, CLE barrier healing was highly accurate for predicting the further course of disease and exceeded endoscopic and histologic remission for predicting a survival free of major adverse outcomes.13 In the case of cancer, physicians improve care by being able to characterize suspicious lesions.11

Cellvizio® mucosal assessment
using Cellvizio® ColoFlexTM UHD Miniprobe
Healthy Mucosa
Criteria: dark goblet cells, regular and narrow vessels surrounding crypts and round crypt structures
Ulcerative colitis
Criteria: mild to moderate increase of capillaries, dilated and distorted capillaries
Dysplastic Mucosa
Criteria: ridged-lined irregular epithelial layer with loss of crypts and goblet cells, Irregular cell architecture with little or no mucin
1. Data from Crohn’s & Colitis Foundation of America, 2014. https://www.crohnscolitisfoundation.org/sites/default/files/2019-02/Updated%20IBD%20Factbook.pdf
2. Bitton A. et al. Predicting relapse in Crohn’s disease: a biopsychosocial model. Gut, 2008.
3. CDC/NCHS national hospital discharge survey: United States, 2010. Centers for Disease Control and Prevention website. www.cdc.gov/nchs/data/nhds/10Detaileddiagnosesprocedures/2010det10_numberalldiagnoses.pdf
4. Liverani E. et al. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol 2016
5. Turcotte JF. et al. Increased Epithelial Gaps in the Small Intestine Are Predictive of Hospitalization and Surgery in Patients With Inflammatory Bowel Disease, Clin tranfl Gastroenterol, 2012
6. Tontini GE. et al. Prediction of clinical outcomes in Crohn’s disease by using confocal laser endomicroscopy: results from a prospective multicenter study. Gastrointestinal Endoscopy. 2017
7. Hundorfean G. et al. Development and Validation of a Confocal Laser Endomicroscopy-Based Score for In Vivo Assessment of Mucosal Healing in Ulcerative Colitis Patients. Inflamm Bowel Dis, 2017.
8. Queneherve L. et al. Quantitative assessment of mucosal architecture using computer-based analysis of confocal laser endomicroscopy in inflammatory bowel diseases. Gastrointest Endosc, (Epub), 2018.
9. Liu J. et al. Personalized Inflammatory Bowel Disease Care Reduced Hospitalizations. Digestive Diseases and Sciences, 2019.
10. Maione F. et al. Confocal laser endomicroscopy in ulcerative colitis: beyond endoscopic assessment of disease activity. Tech Coloproctol, (Epub), 2017
11. Lord R. et al. Colonic lesion characterization in inflammatory bowel disease: A systematic review and metaanalysis. World J Gastroenterol, 2018.
12. Al-Mansour. et al. SAGES TAVAC safety and efficacy analysis confocal laser endomicroscopy. Surg Endosc, 2020.
13. Rath T, et al. Intestinal Barrier Healing Is Superior to Endoscopic and Histologic Remission for Predicting Major Adverse Outcomes in Inflammatory Bowel Disease: The Prospective ERIca Trial. Gastroenterology, 2022.
Cellvizio® I.V.E. with Confocal Miniprobes™ are regulated Medical Devices, CE marked (CE 0459) (Class IIa - NB : G-MED) and FDA cleared. Cellvizio® is a registered trademark and Confocal Miniprobe™ is a trademark of Mauna Kea Technologies. Cellvizio® I.V.E. with Confocal Miniprobes™ is a confocal laser system with fiber optic probes that are intended to allow imaging of the internal microstructure of tissues including, but not limited to, the identification of cells and vessels and their organization or architecture. Once connected to the Cellvizio® I.V.E. system: The GastroFlex™ UHD and ColoFlex™ UHD Confocal Miniprobes™ are intended to allow imaging of anatomical tracts, i.e., gastrointestinal systems, accessed by an endoscope or endoscopic accessories. Please consult labels and instructions for use. These statements and the associated reference to specific clinical studies, are not intended to represent claims of safety or effectiveness for detecting or treating any specific condition or disease state. Rather this information is intended to provide useful reference to selected published literature describing physician experiences with the associated clinical uses. Any diagnostic assessment should always be made by the attending physician, based on the evaluation of all sources of clinical, endoscopic and other relevant information. These statements have not been reviewed, cleared, or approved by the U.S. FDA. The use of this medical device is exclusively reserved for health professionals. Product availability cannot be guaranteed in all countries. For further information, please contact your local sales representative.